NeuroStar Advanced TMS Therapy
How NeuroStar TMS Therapy FAQ's

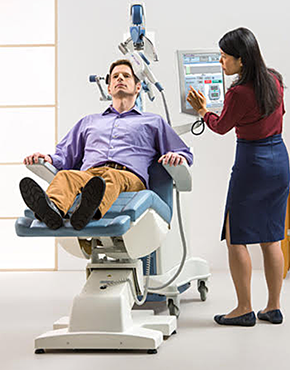
What is Transcranial Magnetic Stimulation?
Transcranial magnetic stimulation, often referred to as TMS is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS is typically used when antidepressant medications haven’t been effective, have ceased working, or as an alternative to medication.
How does TMS work?
TMS involves delivering magnetic pulses to specific parts of the brain.
How long is TMS treatment?
A typical initial course of treatment is about 19-37 minutes daily over 4-6 weeks.
Is TMS therapy covered by my insurance?
A vast majority of commercial and Medicare plans have recognized the effectiveness of treating depression with TMS Therapy and now cover TMS as part of their plans.
Is TMS Therapy a good alternative for patients who cannot tolerate the side effects of antidepressant medications?
TMS does not circulate in the blood throughout the body, so it does not have side effects like weight gain, sexual dysfunction, nausea, dry mouth, sedation, etc. The most common side effects reported during clinical trials were headache and scalp discomfort — generally mild to moderate — occurring less frequently after the first week of treatment.
Is TMS Therapy like other alternative therapies that use magnets to treat some illnesses?
No. TMS Therapy involves a unique method of using pulsed magnetic fields for a therapeutic benefit. The intensity of the magnetic field is similar to that of an MRI. These techniques differ radically from the popular use of low intensity, static magnetic fields. Those products deliver weak and undirected static fields that are not capable of activating brain cells. The activation and stimulation of brain cells is a key part of why TMS is so effective.
Clinical Trials & Academic Studies
1. Carpenter LL, et al. (2012). Transcranial Magnetic Stimulation (TMS) for Major Depression: A Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice. Depres- sion and Anxiety, 29(7):587-596. www.ncbi.nlm.nih.gov/pubmed/22689344
2. George MS, et al. (2010). Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516. www.ncbi.nlm.nih.gov/pubmed/20439832
3. Dunner DL, et al. (2014). A Multisite, Naturalistic, Observational Study of Transcranial Mag- netic Stimulation (TMS) for Patients with Pharmacoresistant Major Depressive Disorder: Durability of Benefit Over a 1-Year Follow-Up Period. J Clin Psychiatry. 75(12):1394-1401. www.ncbi.nlm.nih.gov/ pubmed/25271871
4. O’Reardon JP, et al. (2007). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry, 62(11):1208-1216. www.ncbi.nlm.nih.gov/pubmed/17573044
Learn More About TMS Therapy
NeuroStar TMS Overview
What to Expect
TMS FAQs
TMS Success Stories
Request Information
